Expert Pharmaceutical Translations for Global Drug Development
Medical Translation Services
Break down language barriers and navigate global regulations with ease. Our life science experts deliver precise translations for all your pharmaceutical needs, accelerating drug development and market access worldwide.
Trusted Translations for All Your Needs
Why Choose Our Medical Translation Services?
- ISO 17100 Certified
- Free Delivery
- Quick TAT
Ensure clear and accurate communication for your global pharmaceutical endeavors. We are an ISO 17100 certified translation agency, guaranteeing a rigorous quality management system for all your life science translation needs. Our team of Subject Matter Experts (SMEs) in the pharmaceutical industry delivers meticulous translations, ensuring critical information is conveyed flawlessly. This not only facilitates seamless global drug development but also empowers regulatory compliance across international markets.
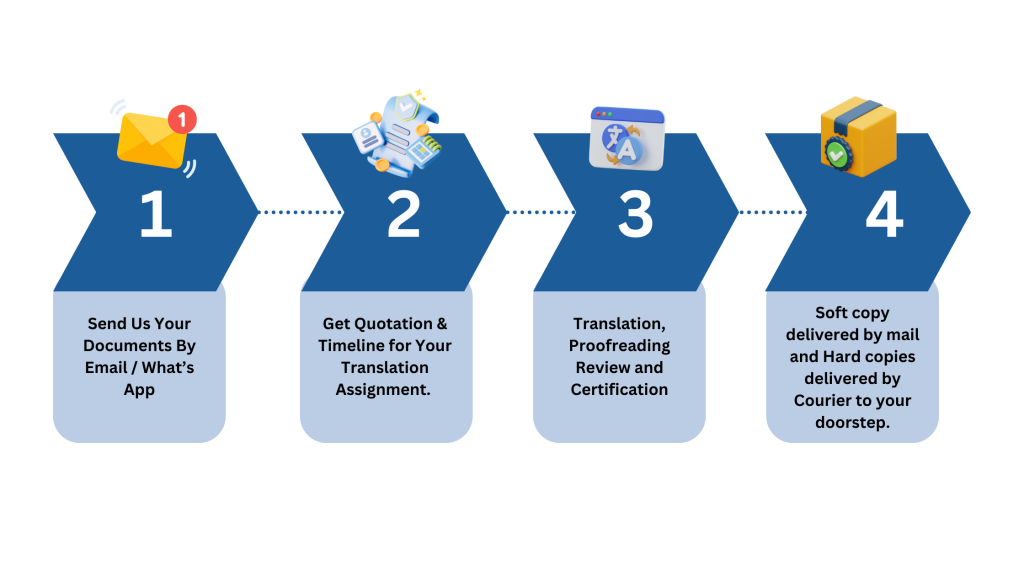
Medical Translations Service Process
Document Categories for Technical Translations
- Informed Consent Forms
- Protocols
- Investigator Brochures
- Clinical study reports (CSRs)
- patient-reported outcomes (PROs)
- Clinical study reports (CSRs)
- adverse event reports
- Drug Press Release
- Medical SOPs
- Marketing Authorization Applications (MAAs)
- Clinical Trial Investigations
- Instructions for use (IFUs)
- Patent applications
- batch records
- Common Technical Documents (CTDs)
We are trusted by Industry Leaders
Our commitment to quality ensures certified translations that are consistently accepted by government offices worldwide. We follow rigorous quality standards to deliver a hassle-free experience for your translation needs.



















Frequently Asked Question
Have questions about our translation services? We’ve compiled answers to some of the most common inquiries to help you get started. If you can’t find what you’re looking for, feel free to contact us directly.
ISO 17100 sets a rigorous quality management standard specifically for translation services in the healthcare field. It ensures accuracy, consistency, and traceability throughout the translation process, crucial for sensitive pharmaceutical documents.
SMEs understand the specific terminology, regulations, and nuances of the pharmaceutical industry. They ensure translations are not only linguistically accurate but also convey scientific concepts and regulatory requirements precisely.
We translate a wide range of documents, including clinical trial materials, regulatory documents, medical writing materials, safety documents, marketing materials, manufacturing documents, and more. (See a full list on our services page).
We take data security very seriously. We have robust non-disclosure agreements (NDAs) in place with all our translators and staff. Additionally, secure file transfer protocols are used to ensure your documents remain confidential throughout the translation process.
Yes, we have a network of qualified translators experienced in numerous languages commonly used in the pharmaceutical industry. Let us know your target language needs, and we’ll find the perfect fit for your project.
Simply contact us through our website or phone number. Our team will be happy to discuss your project requirements and provide a free quote.
Let's Discuss Your Translation Needs
Get a free quote and start your project today. We’re here to help you reach audiences worldwide.